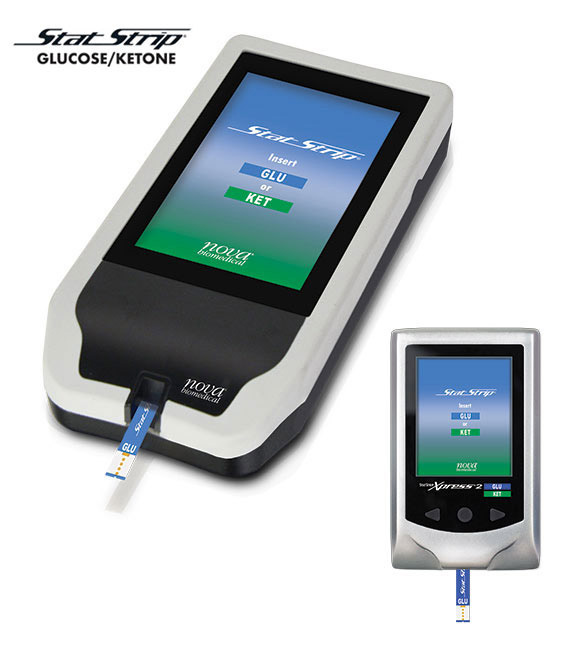
 Next Generation Hospital Glucose/Ketone System
Next Generation Hospital Glucose/Ketone System
Only Glucose Meter FDA-Cleared
for Use with All Critically Ill Patient Samples
• Accuracy proven in study of 1,698
critically ill patients with 257 specific
medical conditions
• No known clinical interferences
• Over
8,000 medications investigated
• Glucose accuracy proven in over
200 publications
Also Measures ß-hydroxybutyrate (ketones)
New Meter Features
Nova’s new Linux-based StatStrip Hospital Glucose/Ketone Meter System has important new features:
- Linux OS with advanced cybersecurity
- Rugged external meter casing
- Larger color touchscreen
- Wireless charging
- Sealed battery casing
- Improved battery charging capacity
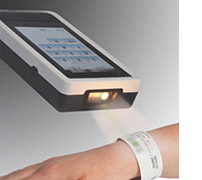
- RFID data entry option
- Extended hematocrit operating range to 5-75%
These features add to StatStrip’s exceptionally accurate glucose measuring technology that has been validated in over 200 peer reviewed publications and extensively proven in hospital critical care settings for over 15 years.
Linux OS with advanced cybersecurity
StatStrip’s Linux based operating system is very secure against computer viruses and other types of malware that affect Microsoft® Windows based operating systems. Linux OS is the platform of choice for over 95% of the world's top one million domains due to its inherent defense against attacks and system compromise.
Rugged, external meter casing
StatStrip’s new meter casing uses a unique soft-touch over-molding material that significantly reduces the potential for damage due to dropping and provides excellent resistance to chemicals and stress cracking.
New ketone blood test*
Insert a ketone strip and the StatStrip Glucose/Ketone meter automatically converts to the ketone measuring mode. Ketone testing aids in managing diabetic ketoacidosis (DKA), a potentially life-threatening complication of both type 1 and type 2 diabetes.

 RFID data entry option
RFID data entry option
StatStrip users can now easily enter operator ID and patient ID
through RFID / Near Field Communication (NFC: 13.56MHz), barcode scanning,
or touchscreen keyboard entry.
Improved, illuminated barcode scanner
A new barcode scanner reads both 1D and 2D barcodes,
delivers excellent
barcode scanning speed and acuity, and
accommodates a broad range of barcode
types. The lighted
barcode scanner brightly illuminates the
barcode label for
easier scanning.
Touchscreen Entry
StatStrip's 4-inch color touchscreen screen provides a large
onscreen keyboard for gloved hand entry of alphanumeric
operator and patient ID.
Improved battery charging capacity
A new battery with 75% more capacity extends meter use-time between charges.
Wireless charging
Wireless meter charging provides faster, easier and safer meter charging in a smaller package. The battery is charged in the meter’s sealed compartment and the charger automatically shuts off when the meter is fully charged, reducing energy use and preventing battery overheating.
Expanded wireless connectivity network
A single NovaNet server can support wireless connectivity for all meters and Nova analyzers throughout multiple facilities.
*The Next Generation StatStrip Glucose/Ketone system has received the CE mark and is not yet available in the US.
Learn more
To learn more about how StatStrip Glucose/Ketone can bring safe, accurate critical care testing to the bedside, contact your Nova representative or call Nova at 800-458-5813.
For more product information, click here or contact Nova Biomedical at:
Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800
For Nova Biomedical Sales inquiries, reach us at 1-800-458-5813.
For Technical Support, reach us at 1-800-545-NOVA (6682).
To place an order, call 1-800-822-0911 or email novaorders@novabio.com
Specifications
Next Generation Hospital Glucose/Ketone System

Weight: 0.47 lb (215 g)
Size: 6.23 in x 3.04 in x 1.11 in (158 mm x 77 mm x 28 mm)
Meter Data Storage:
Patient Tests: 1,500 Tests
QC Tests: 200 Tests
Users: 8,000 Users
Connectivity:
Data Output: RJ-45 Ethernet Port
Protocol: TCP/IP Ethernet 100 Mbit
Standard: POCT1-A2 Compliant
Operating Ranges:
Temperature Glucose: 41˚F-104˚F (5˚C-40˚C)
Temperature Ketone: 34˚F-104˚F (1˚C-40˚C)
Altitude: Up to 15,000 feet (4,572 meters)
Humidity: 10% to 90% relative humidity
Battery Information:
Type: 3.6 V Li Polymer Rechargeable Battery
Wireless Specifications:
Wireless Standard: IEEE 802.11a/b/g/n, Ethernet: IEEE 802.3u
Data Rate: Up to 54Mbps
Modulation: 64QAM, 16QAM, BPSK, QPSK, DBPSK, DQPSK and CCK
Frequency Range: 2.4 and 5Ghz supported
Wireless Security: WEP, WPA, WPA2, RADIUS, 802.1x
Encryption Types: RC4, TKIP, AES, PSK, EAP-FAST, EAP-TLS, EAP-TTLS, PEAP-GTC,
PEAP-MSCHAPv2, PEAP-TLS, LEAP
StatStrip Glucose Test Strips
Test Measured:
Blood Glucose, Hematocrit Corrected
Test Reported:
Glucose
Test Time:
6 Seconds
Test Strip Volume:
1.2 µL
Test Methodology:
Electrochemistry
Sample Types & Operating Modes:
Whole Blood: Arterial, Venous, Capillary, Neonatal
Glucose Measurement Range:
0.6-33.3 mmol/L (10-600 mg/dL)
Interferences, Measured and Corrected:
Hematocrit, Ascorbic Acid, Uric Acid, Acetaminophen, N-acetylcysteine, Bilirubin, Maltose, Galactose, Oxygen
Test Strip Stability:
24 months from date of manufacture 6 months open-vial stability
StatStrip Xpress2 Glucose/Ketone Meter

Weight: 0.2 lb (75 - 78.5 g)
Size: 3.6 in x 2.4 in x 0.9 in (98 mm x 61 mm x 23 mm)
Meter Data Storage:
Patient & QC Tests: 400 tests total (FIFO)
Connectivity:
Data Transfer: Strip Port Connection to USB
Data Program: Nova Microsoft-Excel based data transfer software
Operating Ranges:
Temperature Glucose: 41˚F-104˚F (5˚C-40˚C)
Temperature Ketone: 34˚F-104˚F (1˚C-40˚C)
Altitude: Up to 15,000 feet (4,572 meters)
Humidity: 10% to 90% relative humidity
Battery Information:
2 AAA batteries
StatStrip
Ketone Test Strips

Test Measured:
Blood Ketone, Hematocrit Corrected
Test Reported:
Ketone
Test Time:
10 Seconds
Test Strip Volume:
0.8 µL
Test Methodology:
Electrochemistry
Sample Types & Operating Modes:
Whole Blood: Venous, Capillary
Ketone Measurement Range:
StatStrip Hospital Meter 0.1-7.0 mmol/L
StatStrip Xpress 2 Meter 0-8.0 mmol/L
Interferences, Measured and Corrected:
Hematocrit, Ascorbic Acid, Uric Acid, Acetaminophen, Bilirubin, Oxygen Maltose, Galactose, Oxygen
Test Strip & QC Stability:
24 months from date of manufacture 3 months open-vial stability
Certifications and Compliance: Nova Biomedical complies with the FDA Quality Systems Regulations and is certified to EN ISO 13485:2016. Complies to IVDD. Tested according to: EN 61010-1:2010, EN 61010-2-101:2015, EN 60825-1/A1:2014. Certifications and compliances applicable in countries where regulations require.
Specifications current as of revision date.
For more product information, click here or contact Nova Biomedical at:
Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800
For Nova Biomedical Sales inquiries, reach us at 1-800-458-5813.
For Technical Support, reach us at 1-800-545-NOVA (6682).
To place an order, call 1-800-822-0911 or email novaorders@novabio.com